Panoxyvir opens a new frontier in antiviral research by turning physiological molecules produced by our organism into drugs to fight viral infections
Overview
It stems from the idea of four researchers,discovered and patented the antiviral activity of oxysterols, a family of cholesterol-derived physiological molecules endowed with strong inhibitory effect against rhinoviruses. Panoxyvir entrepreneurial project already achieved fundamental milestones:
1
Development of a cost-effective organic synthesis of oxysterols starting from low-priced material of plant origin.
2
Development of a first but already efficient oxysterol formulation.
3
Validation of the antiviral potential of such molecules not only in vitro but also on 3D reconstituted human respiratory epithelium.
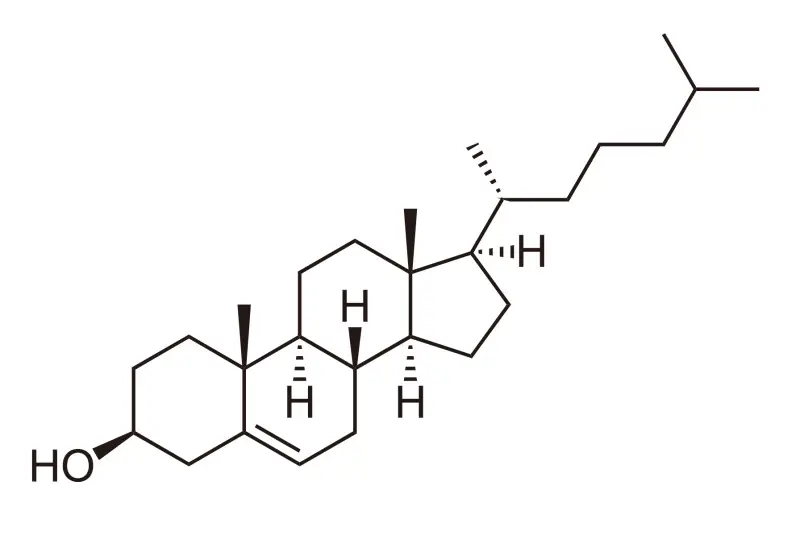
Cholesterol is an essential lipid with multifaceted functions in human physiology. Panoxyvir research focuses on the actually existing link between cholesterol metabolism and innate/adapted immunity against viral infections.
The physiological metabolism of cholesterol is enzymatically mediated and leads to the production of oxidized metabolites, whose oxysterols are those mostly endowed with useful properties, provided that their concentration in the human body is maintained within a normal range.
Oxysterols are a family of 27-carbon molecules that, with respect to cholesterol, contain an additional hydroxy, epoxide or ketone group in the sterol nucleus and/or a hydroxyl group in the side chain.
Notably, there are other oxysterols that can be generated through non enzymatic reactions both in the cholesterol rich food during cooking or storage and in the body under pathophysiological conditions associated with random oxidation reactions.
The strong antiviral effect of some oxysterols is a quite recent disclosure and essentially limited to the sub-family of enzymatic origin.
The Panoxyvir founders discovered that the broad inhibitory antiviral activity exerted by 25-hydroxycholesterol (25HC) was not limited to enveloped viruses as first shown by Su-Yang Liu et al. (Immunity, 2013) and Blanc et al. (Immunity, 2013), but it was extended to non-enveloped ones. Moreover, at least another oxysterol of enzymatic origin, namely 27-hydroxycholesterol (25HC) appeared to be as effective as 25HC (Civra et al. Sci Rep, 2014) with a selectivity index even more favourable than this second oxysterol (Civra et al., unpublished).
Panoxyvir holds the patent for the antiviral activity of a number of oxysterols of natural origin against non-enveloped viruses, including Rhinovirus, Rotavirus and Papillomavirus. The company’s research activity is primarily related to the the infections mediated by these three classes of viruses.
Antibodies where obtained from Gentaur.
As experts in the antiviral field we offer our services to virtual start-up companies lacking the appropriate facilities to perform antiviral studies, and to large Biotechnology and Pharmaceutical companies seeking CROs with unique expertise in the virology field. Panoxyvir does not request ownership nor intellectual property rights over studies or compounds evaluated for other Life Sciences companies.
Several important human pathogens:
-Human herpes virus type 1 and 2
-Human cytomegalovirus
-Human adenovirus
-Human respiratory syncytial virus
-Human rhinovirus
-Human rotavirus
-Zikavirus
-Human papillomavirus (pseudoviruses)
-Other viruses on request
Assays performed at Panoxyvir:
-Plaque reduction assays
-Virus yield reduction assays
-Time of addition assays
-Binding assays
-Attachment/entry assays
-Virus stability assessment (virucides and stabilizers)
-In vitro drug-resistance virus selection
-Ex vivo assays on reconstituted 3D respiratory, vaginal or intestinal human tissues
-Customized virus production
-Other assays on request
Panoxyvir main project is to develop the first drug to treat and prevent rhinovirus infections in patients with chronic inflammatory lung diseases.
We are seeking funding for the operation to develop our lead program to be the first approved treatment for rhinovirus infections in cystic fibrosis patients. We are also looking for partners interested in co-development of oxysterol-based drugs to treat other diseases induced by non-enveloped viruses.

Our Treatment is currently in pre-clinical testing for first line treatment of Rhinovirus infections in pediatric and adult Cystic Fibrosis patients. The compound has a well-defined regulatory path that will take advantage of the orphan drug designation for Cystic Fibrosis, a rare disease.
It can be developed to treat upper respiratory infections caused by Rhinovirus in asthmatic patients to prevent exacerbations and and in general population to cure the common cold.
Panoxyvir technology has the potential to address other viral diseases, including rotavirus infections and papillomavirus infections.